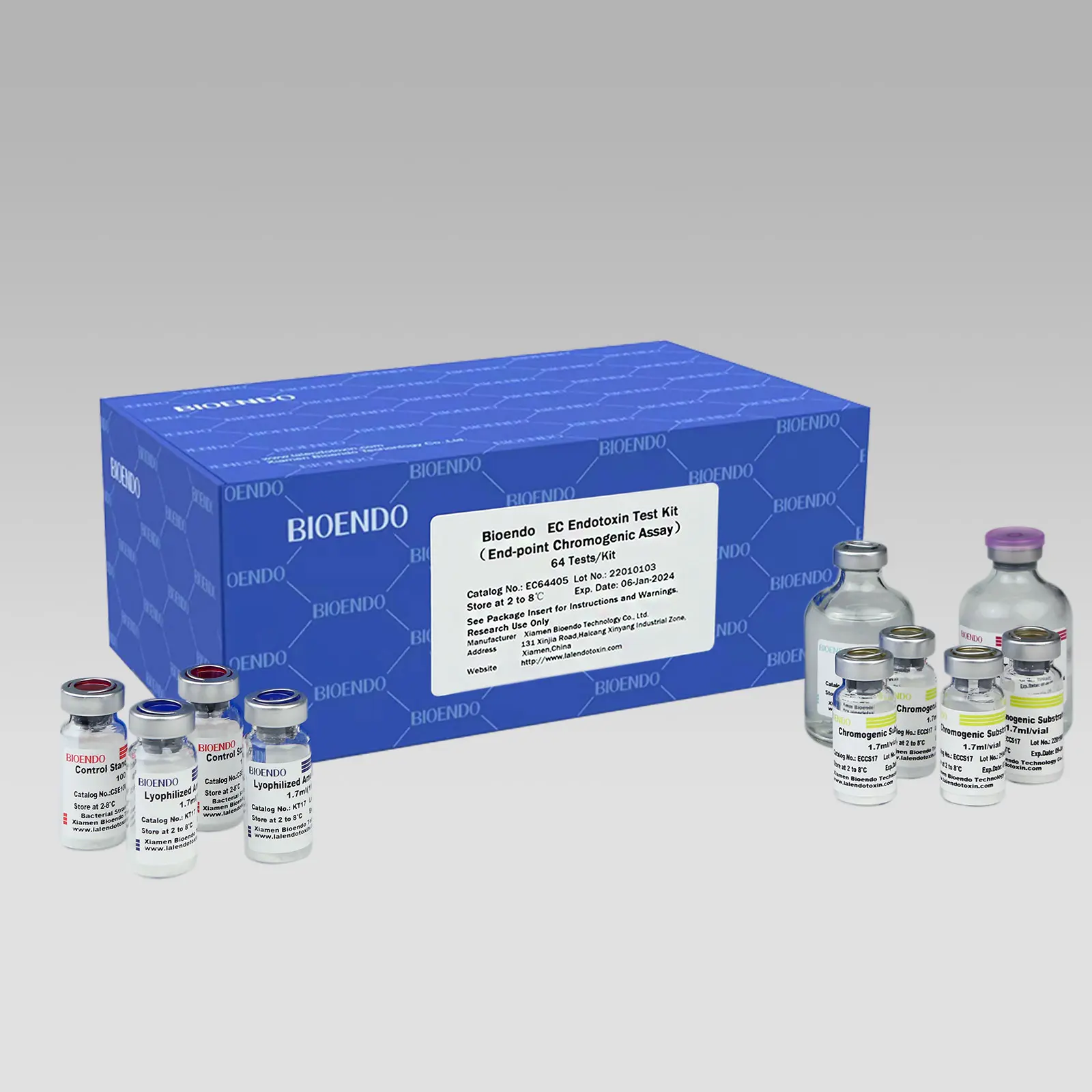
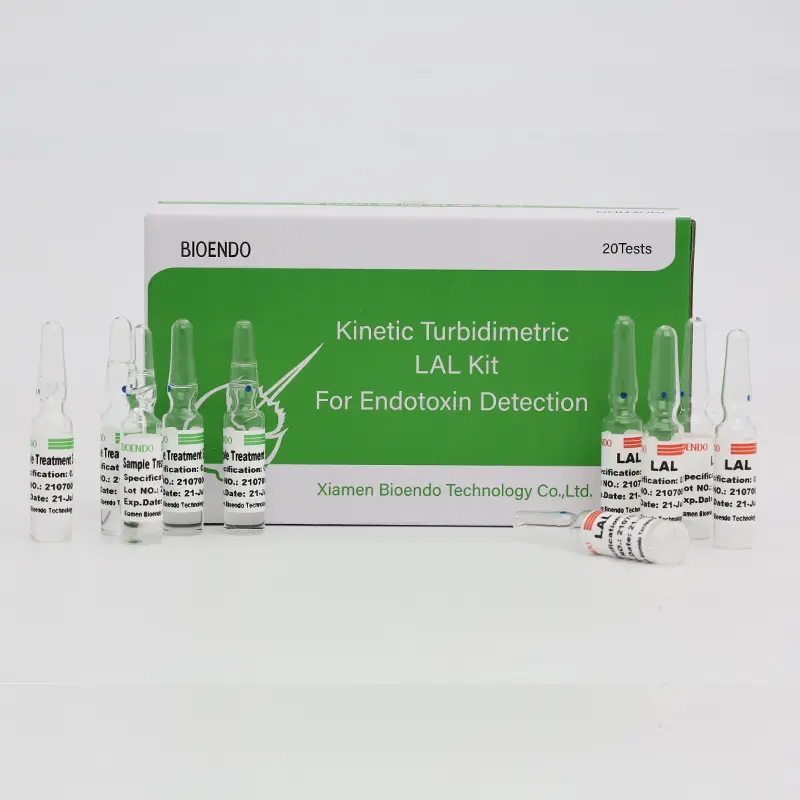
Kinetic Turbidimetric Amebocyte Lysate Vial is developed based on the principle that the time needed to reach a certain absorbance increase (onset OD), i.e. onset time, is negatively correlated with the endotoxin concentration.
PRODUCT INTRODUCTION
Kinetic Turbidimetric Amebocyte Lysate Vial is developed based on the principle that the time needed to reach a certain absorbance increase (onset OD), i.e. onset time, is negatively correlated with the endotoxin concentration. Sensitivity could reach 0.005 EU/ml, and the detection could reach four orders of magnitude. It is specially suitable for pharmaceuticals industry to monitor endotoxin concentration.
Specifications
Assay range: 0.005 -50 EU/ml; 0.01 – 10 EU/ml
Application
End-product endotoxin (pyrogen) qualification, Water for injection endotoxin assay, raw material endotoxin testing or endotoxin level monitoring during manufacturing process for pharmaceutical companies or medical devices manufacturers.
Order information
| Catalog No. | ml/vial | Tests/Vial | Vials/Pack | Sensitivity EU/ml |
| KT17 | 1.7 | 16 | 10 | 0.01 - 10 EU/ml |
| KT17S | 1.7 | 16 | 10 | 0.005 - 5 EU/ml, 0.01 - 10 EU/ml |
| KT52 | 5.2 | 50 | 10 | 0.01 - 10 EU/ml |
| KT52S | 5.2 | 50 | 10 | 0.005 - 5 EU/ml, 0.01 - 10 EU/ml |